Overview
This post summarises the results from the project ‘Using daily heart rate variability measures to identify the onset and severity of COVID-19’. The project was run by HRV Fit Ltd, and funded by Innovate UK, and aimed to explore whether changes in HRV are related to the timing and severity of COVID-19 symptoms. The project asked users of apps recording daily Heart Rate Variability (HRV) who had confirmed or suspected COVID-19 to submit their HRV data and complete a questionnaire about their COVID-19 symptoms. We would like to take the opportunity to thank everyone who took part in the project and hope that you find the results interesting.
Background
During infection, the body detects the presence of foreign cells (such as viruses) and elicits a protective response via the immune system. This response leads to a process known as inflammation, which acts to clear the infectious stimuli and initiate tissue healing. Although the inflammatory response helps to remove the stimuli, if the response is too great it can lead to harmful effects.
Studies looking at the level of inflammation (measured via so-called ‘inflammatory markers’) have shown differing levels of inflammation relate to outcomes of COVID-19 infection, with higher levels of inflammation associated with worse outcomes (Zhou et al., 2020).
Vagal activity (part of the body’s autonomic nervous system) is responsible for regulating changes in heart rate and is also part of a complex process that regulates inflammatory responses. The vagal pathway acts to inhibit inflammation, via a specific anti-inflammatory pathway (see Huston and Tracey, 2011 for review). HRV is a non-invasive index of vagal activity; however, the question remains if HRV could provide a window into the body’s response to COVID-19 infection.
Results
Nineteen people took part in the study. The average age (± standard deviation – a measure of the variance around the average) was 50 ± 10 years old, with 4 females and 15 males. There were 5 confirmed COVID-19 cases (positive antigen or antibody test) and 15 suspected COVID-19 cases (based on self-reported symptoms). Seventeen participants managed their symptoms at home, whilst two visited a hospital, but did not stay overnight.
Changes in HR and HRV relative to symptom onset
The results from the study show that the heart rate (HR) and HRV responses to confirmed or suspected COVID-19 infection can be highly variable between individuals, both in terms of the size and direction of changes. We developed a new index, named the ‘infection index’, which incorporates both HR and HRV. A change in the infection index was seen in 63.2% of participants between day -12 and day +3, relative to symptom onset (day 0). However overall, this was not significantly different from random chance. The direction of the change also varied between individuals; where the infection index increased in some but decreased in others.
Changes in HR and HRV relative to symptom severity
The volunteers participating in the study experienced relatively mild symptoms, with nobody needing to stay overnight at a hospital. Thus, we used symptom duration as a marker of symptom ‘severity’. There seemed to be a trend for longer lasting changes in HR and HRV in those who had symptoms for longer; however, this varied a lot between individuals. The figures below show the change in HR from baseline for those who had symptoms for 1 to 20 days (Figure 1A, 16 people) and those who had symptoms for over 20 days (Figure 1B, 3 people).
Analysis further showed that the time taken for the infection index to reach a minimum value after symptom onset is related to how long symptoms last for. The magnitude of the infection index decrease after symptom onset is also related to which symptoms individuals’ experience. A large change makes it statistically more likely that a participant experienced a productive cough and/or altered sense of smell/taste.
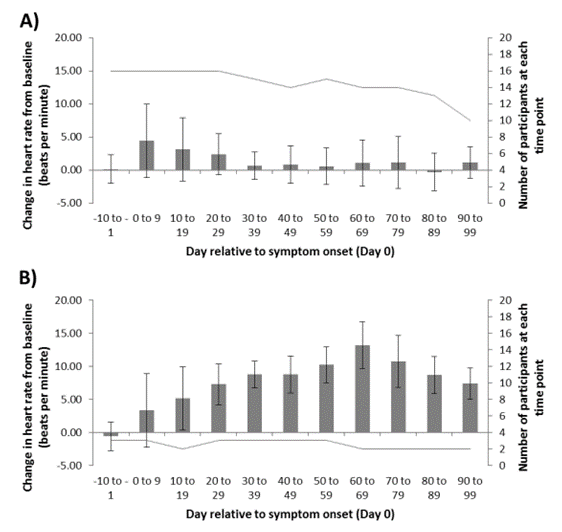
Figure 1. Change in heart rate (HR) relative to baseline for individuals with 1-20 days of COVID-19 symptoms (Panel A), and those with over 20 days of symptoms (Panel B). Data displayed as mean ± standard deviation (a measure of the variance of the data).
Symptoms
A moderately strong relationship was found between several pairs of symptoms. This means that, overall, people who had one of these symptoms were also statistically significantly more likely to experience some other symptoms too. Participants who experienced chest tightness were likely to experience loss of appetite, muscle ache/pain and productive cough too. Participants who experience productive cough were likely to experience fatigue, chest tightness and sore throat.
Discussion
The study demonstrated highly variable responses in HR and HRV relative to COVID-19 symptom onset. In light of the now emerging evidence of a the extremely wide range of symptom severity (none to requiring ICU admission) in COVID-19 infection, this is perhaps unsurprising. There could also be several study-specific reasons for the heterogeneity, including the small group studied; potential inclusion of cases where suspected symptoms may not have been due to COVID-19; inclusion of only mild COVID-19 cases; or the population studied not being representative of the general population, e.g., it is likely that the group studied had higher fitness levels.
We were unable to detect changes in HR or HRV prior to symptom onset, but some interesting trends were observed in relation to how responses vary between those who have symptoms for different durations. Individuals who had longer lasting symptoms tended to show a sustained decrease in HRV and increase in HR compared to those with a shorter symptom duration. Furthermore, a later decrease in HRV/HR after symptoms started was associated with longer lasting symptoms. This could help to provide some insight into how the autonomic nervous system is affected by COVID-19 infection, with further work needed to investigate if changes are different in people who then go on to experience so- called ‘long COVID’ (see Mahase, 2020 for more information on ‘long-COVID’).
We found some interesting ‘clusters’ of symptoms that were likely to occur together. Similar clusters have also been found in larger studies focusing on symptoms only (Sudre et al., 2020). Some of the clusters found in this study are similar to clusters already identified, however none are identical. This is likely due to the smaller number of participants in this study.
What is next?
We intend to publish a case study describing one ithlete user’s experience with long-lasting COVID19 symptoms, and the changes they saw in their resting HR and HRV. This case study will help open up the conversation with clinicians and researchers about how the autonomic nervous system might be impacted in (some) people with COVID-19; what this means for future patient care, as well as how this could support new monitoring approaches using technology like ithlete. Further research would also help to confirm if those living with ‘long-COVID’ are affected more than those who recover quickly.
We aim to add the new ‘infection index’ we developed during this study to ‘ithlete Pro’ in the future, however at this stage it won’t be able to tell you anything about COVID-19, but we hope it will be another useful training-support tool.
Further work on the link between HR and HRV is now also being undertaken by other companies, using ‘wearables’ like smart watches and smart rings. However, no HR or HRV based system has yet been proven to be able to detect the pre-symptomatic onset of COVID-19, despite promising progress being made (see pre-print (awaiting peer-review) by Natarajan et al., 2020).
The data we gathered in the study provided some promising preliminary data about the potential use of HRV in detecting the onset of COVID-19 symptoms, as well as in monitoring changes in symptoms over time. The ‘proof of concept’ provided by our study justifies further research, in order to test whether the trends we observed can be leveraged to provide predictive indicators that can be harnessed to better manage recovery from COVID-19. Therefore, we are keeping the survey open for anyone else who wants to take part, with an updated Information Sheet and Privacy Notice.
References
Huston, J. M., & Tracey, K. J. (2011). The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. Journal of internal medicine, 269(1), 45-53.
Mahase, E. (2020). Covid-19: What do we know about “long COVID-19”?. BMJ, 370:m2815, doi: https://doi.org/10.1136/bmj.m2815
Natarajan, A., Su, H. W., & Heneghan, C. (2020). Assessment of physiological signs associated with COVID-19 measured using wearable devices. medRxiv. doi: https://doi.org/10.1101/2020.08.14.20175265
Sudre, C. H., Lee, K., Lochlainn, M. N., Varsavsky, T., Murray, B., Graham, M. S., … & Drew, D. A. (2020). Symptom clusters in Covid19: A potential clinical prediction tool from the COVID Symptom study app. MedRxiv. doi: https://doi.org/10.1101/2020.06.12.20129056
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., Xiang, J., Wang, Y., Song, B., Gu, X. and Guan, L., 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 395(10229), 1054-1062.

Dear,
I am Alexandre Medeiros (Professor of Federal University of Ceara – Brazil). We are conducting an study with people that are with COVID. We would like to use ithlete to performed RHV. Is it possible to tranfer the data to Kubio? The app is free?
Beste regards,
Alexandre medeiros.
Dear Alexandre,
Thanks for your interest in ithlete HRV. The HRV (20x LnRMSSD) and HR calculations have been independently validated but we don’t export raw RR intervals to Kubios.
You can transfer the data to our Team system, or by email from the mobile app.
We do offer special pricing for research studies.
best regards,
Simon Wegerif.
Duke University is also busy with a study on this relation.
This is good to hear, we will be interested to see what is found.